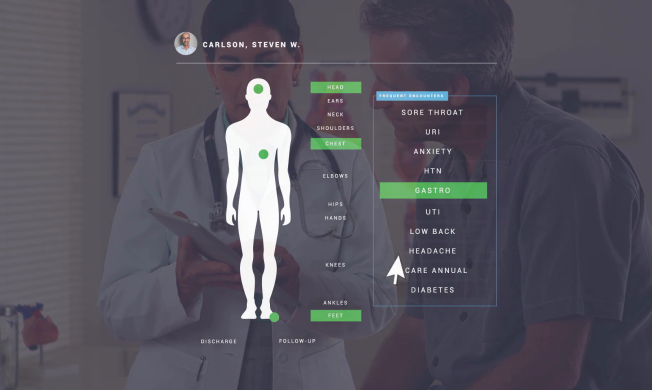
See Greenway Health in Action!
EHR and Practice Management solutions designed for every aspect of your practice — from front office to clinical to back office — Greenway Health gives you the tools for success. Watch this short video to see how partnering with Greenway can streamline office workflows, boost revenue-generating opportunities, and improve the patient experience.
Copy video link to clipboard: